Thoracic Aorta
Aorta, the great vessel of the heart, brings an oxygenated blood to the whole organism. Except from the “pipeline” function, it works as a secondary pump (so called Windkessel effect, caused by elasticity of aortic wall) that is able to maintain the blood pressure and organ perfusion in diastole. There are baroreceptors in the aortic wall which influence the heart rate and systemic vascular resistance during blood pressure changes. The normal aortic diameter does not exceed 40 mm and it gets narrower distally. Anatomically, the aorta is divided into thoracic and abdominal part by diaphragm. The thoracic aorta is further divided into:
- aortic root (the beginning are the aortic valve anulus and cusps, followed by sinuses of Valsalva and sinotubular junction)
- ascending aorta (beginning above the sinotubular junction up to the offset of the brachiocephalic trunk)
- aortic arch (from the brachiocephalic trunk to the left subclavian artery offset)
- descending thoracic aorta (from the left subclavian artery to the diaphragm)

The diseases of the thoracic aorta include a wide range of units, the most important for surgery are: thoracic aortic aneurysms, acute aortic thoracic syndromes (acute aortic dissection, intramural haematoma, penetrating aortic ulcer, aortic pseudoaneurysm). The independent categories are thoracic aortic injury and aortic tumors. Congenital anomalies (aortic coarctation, transposition of great arteries, aneurysm of the sinuses of Valsalva etc.) are discussed elsewhere.
Thoracic Aortic Aneurysm
Definition
 Aortic aneurysm is severe localized dilation of aortic diameter by at least 50% of an expected diameter. A normal ascending aortic size is up to 3,9 cm in men and 3,7 cm in women, descending aortic size should be below 3,0 cm in men and 2,6 cm in women. Aneurysm can occur in any thoracic aortic region, there can be more regions diseased, the thoracic aorta can be affected as a whole, too. The wall of a true aortic aneurysm consists of all the three aortic wall layers.
Aortic aneurysm is severe localized dilation of aortic diameter by at least 50% of an expected diameter. A normal ascending aortic size is up to 3,9 cm in men and 3,7 cm in women, descending aortic size should be below 3,0 cm in men and 2,6 cm in women. Aneurysm can occur in any thoracic aortic region, there can be more regions diseased, the thoracic aorta can be affected as a whole, too. The wall of a true aortic aneurysm consists of all the three aortic wall layers.
Etiology
The possible causes of aneurysmal dilation of the thoracic aorta are:
- atherosclerotic degeneration of the aortic wall (risk factors are male sex, old age, smoking, hypertension, dyslipidemia, family history of the disease etc.)
- congenital connective tissue disorders (Marfan syndrome, Ehlers-Danlos syndrome type IV = vascular type, Loeys-Dietz syndrome, aortic wall disease in bicuspid aortic valve)
- status post acute aortic dissection, chronic thoracic aortic dissection
- chest trauma
- pseudoaneurysms in aanstomoses after vascular reconstruction surgery
- autoimmune aortitis (giant cell arteritis, Takayasu‘s arteritis)
- infective aortitis (Staphylococcus, Salmonella, Mycobacteria, Treponema pallidum)
Symptoms
The symptoms of a slowly growing thoracic aortic aneurysm are poor - the patients are usually asymptomatic. Rarely the following occur:
- pain: precordial (ascending aortic aneurysm), propagation into the neck and mandible (arch aneurysm), upper back pain, usually between the shoulder blades (descending thoracic aortic aneurysm), lower back pain (thoracoabdominal aneurysm)
- compression of the surrounding organs: hoarseness (irritation of the left-sided recurrent laryngeal nerve), superior vena cava syndrome
- signs of complications: embolization of blood clots from the aneurysmal sac (stroke, limb ischemia, visceral ischemia), aortic dissection or rupture (extreme chest pain, loss of consciousness, shock)

Diagnosis
Physical examination is non-diagnostic. There may be a diastolic murmur heard above the aorta in concurrent aortic valve regurgitation, together with Corrigan pulse and de Musset’s sign.
Chest X-Ray may show a mediastinal widening.
Echocardiography is able to assess the presence and severity of root, ascending aortic, and arch aneurysms. The descending aorta is less accesible.
The sovereign diagnostic modalities are computed tomography (CT) and magnetic resonance imaging (MRI), both with or without contrast medium. A 3D reconstruction is beneficial for exact size measurement. Both modalities are able to evaluate the presence of an intramural haematoma, aortic ulcer, or disease of aortic branches. CT is preferred in acute conditions, on the other hand, MRI is suitable for long-term follow-up in young people (there is no ionizing radiation). Aortography is not sufficient for disease evaluation, it provides only 2D picture of aortic lumen. Vessel wall disease and eventual thrombotic lining of the arterial wall cannot be assessed.
Therapy
Conservative treatment comes into consideration in patients whose aortic diameter does not reach the interventional treatment threshold. The keystone of the conservative management is adequate correction of arterial hypertension, dyslipidemia, lifestyle, avoidance of heavy weight-lifting and contact sports. Regular surveillance is an obviosity.
An intervention is always indicated in symptomatic patients. In asymptomatic patients it may be considered when the risk of complications outweighs the surgical risk. This threshold is 55 mm in the maximal aortic diameter in otherwise healthy patient. In case of a patient with bicuspid aortic valve and other risk factors (hypertension, family history of aortic dissection, coarctation of the aorta, significant increase in aortic diameter on yearly control), or in a patient with Marfan syndrome, the threshold goes down to 50 mm. When the patient with Marfan syndrome has the aforementioned risk factors, or when the aortic valve replacement is planned, the aortic intervention is indicated above 45 mm.
Surgical treatment is a gold standard treatment for severe aneurysmal dilation of the thoracic aorta. The principle of surgery is to replace the diseased portion of aorta by vascular tube graft. The operation has some specifications according to the location of the disease:
- Anuloaortic ectasia
- Aneurysm of the ascending aorta
- Aneurysm of the aortic arch
- Aneurysm of the descending thoracic aorta
1. Anuloaortic ectasia
Dilation in the level of aortic anulus, sinuses of Valsalva and sinotubular junction. It may be treated by composite prosthetic replacement (Bentall procedure), replacement by xenograft, or one of aortic valve sparing procedures (Yacoub or David procedure). The decision depends on the aortic valve condition, aortic root anatomy, patient’s life expectancy, anticoagulation circumstances and last but not least, experience of the surgical team. The procedures are described in detail in the chapter of aortic valve disease.


2. Ascending aortic replacement
It comes into consideration in a dilation of the ascending aorta above the level of sinotubular junction. It is therefore not required to handle with aortic root and coronary arteries. The procedure is called supracoronary ascending aortic replacement. From technical point of view, it is more difficult to introduce arterial cannula for extracorporeal circulation, one has to place it distally, into the aortic arch (or eventually into a peripheral artery - femoral or axillary). After cross-clamping the aorta right before the brachiocephalic trunk, the cardioplegia is administered. Dilated aortic portion is excised and replaced by dacron tube graft.
In a high risk patient cohort (old age, comorbidities, complex surgery) it is possible to use some of the less invasive techniques - external wrapping or reduction aortoplasty of the ascending aorta.
In case the aortic dilation extends up to the arch, the systemic cooling of the patient is required. The affected aortic wall is excised in deep hypothermic circulatory arrest and an open distal anastomosis in the aortic arch is performed (so called hemi-arch procedure).


3. Aortic arch replacement
Surgical treatment of aortic arch aneurysms is technically very demanding. There are a few possibilities here. In most of the cases, the “hemi-arch replacement” is sufficient. The convexity of the arch and the supraaortic vessels are preserved in situ, only the concavity of the arch is being replaced together with the ascending aorta. On the convex side, the replacement ends before the offset of the brachicephalic trunk. On the concavity the replacement goes down to the level of left subclavian artery.


 In patients with extreme dilation of the whole aortic arch, that often continues to the descending part, the “total-arch replacement” comes into consideration. The problematic part of this is the reimplantation of the supraaortic vessels. Until recently the preferred technique was an island technique seu Carrel where one common button of all three supraaortic vessels is reimplanted together with the surrounding aortic wall. Nowadays there are special aortic vascular grafts with prefabricated branches for these vessels, it is therefore possible to suture them separately. This has proved advantageous particularly for reduction of the risk of embolizations and stroke.
In patients with extreme dilation of the whole aortic arch, that often continues to the descending part, the “total-arch replacement” comes into consideration. The problematic part of this is the reimplantation of the supraaortic vessels. Until recently the preferred technique was an island technique seu Carrel where one common button of all three supraaortic vessels is reimplanted together with the surrounding aortic wall. Nowadays there are special aortic vascular grafts with prefabricated branches for these vessels, it is therefore possible to suture them separately. This has proved advantageous particularly for reduction of the risk of embolizations and stroke.
 When the aneurysm continues down to the descending aorta, it is possible to use one of the elephant trunk (ET) techniques. Together with the total-arch replacement, there is a part of the prosthesis left continuing inside the descending aorta. In conventional ET, the distal part of the prosthesis “soars” freely in the descending aorta where it is ready for a second-stage procedure (descending aortic replacement or implantation of a stentgraft). In frozen ET technique, a stentgraft is being implanted intraoperatively in an antegrade fashion into the descending aorta in order to stabilize the aortic wall. There is no second-stage procedure required, however, it has some technical limitations and risks.
When the aneurysm continues down to the descending aorta, it is possible to use one of the elephant trunk (ET) techniques. Together with the total-arch replacement, there is a part of the prosthesis left continuing inside the descending aorta. In conventional ET, the distal part of the prosthesis “soars” freely in the descending aorta where it is ready for a second-stage procedure (descending aortic replacement or implantation of a stentgraft). In frozen ET technique, a stentgraft is being implanted intraoperatively in an antegrade fashion into the descending aorta in order to stabilize the aortic wall. There is no second-stage procedure required, however, it has some technical limitations and risks.
Operations of the aortic arch are technically very demanding and they can be performed only in a bloodless operative field in deep hypothermic circulatory arrest. Cerebral protection is a very important part of the procedure, there are some techniques to maintain brain perfusion during the surgery - retrograde, or currently mostly antegrade. After establishment of the circulatory arrest, the arterial cannulas of the extracorporeal circulation are inserted into the carotid arteries. By this means the adequate cerebral perfusion is ensured until the surgeon performs a reconstruction.
4. Descending aortic replacement
 A suitable approach for the descending thoracic aorta is a left-sided thoracotomy (through 4th - 7th intercostal space). To maintain peripheral organ perfusion, a left-heart bypass may be used. The blood is derived from the left atrium, through a pump, into the distal aorta or a femoral artery. An alternative is to use peripheral extracorporeal circulation (using femoral artery and vein).
A suitable approach for the descending thoracic aorta is a left-sided thoracotomy (through 4th - 7th intercostal space). To maintain peripheral organ perfusion, a left-heart bypass may be used. The blood is derived from the left atrium, through a pump, into the distal aorta or a femoral artery. An alternative is to use peripheral extracorporeal circulation (using femoral artery and vein).
The diseased portion of the descending aorta is excluded from the circulation by cross-clamping. Then it is excised and replaced by a tube graft. The most severe complication of this procedure is an irreversible paraplegia due to ischemic spinal cord injury. As a prevention of this, these protective mechanisms are being used:
- systemic cooling
- preventive cerebrospinal fluid drainage
- preservation of the intercostal branches Th8 - L1 and sewing them to the graft (in this region is very probably the arteria radicularis magna, artery of Adamkiewicz, that plays an important role in blood supply of the spinal cord)
The risk of paraplegia is currently between 6-8%. The early operative mortality is similar.
Endovascular therapy, or Thoracic EndoVascular Aortic Repair (TEVAR), represents a modern and minimally invasive approach in treatment of thoracic aortic disease. The principle is to exclude the aortic lesion from the circulation by implanting a membrane-covered stent (=stentgraft) across it, in order to prevent further enlargement and ultimate aortic rupture. It is delivered into the position in a retrograde fashion through a peripheral arterial access under skiascopic control. An important requirement is a sufficient and safe proximal landing zone of the stentgraft above the diseased portion of the aorta. It is often used to overlap descending aortic aneurysms. When there is an effort to treat arch lesions, a preprocedural revascularization of supraaortic vessels is required (limited revascularisation or total arch “debranching” - all the vessels are transposed onto the ascending aortic prosthesis). These procedures are called hybrid - a combination of endovascular and limited surgical approach. Another way to treat arch aneurysms is an introduction of fenestrated or branched stentgrafts, or eventually so called “chimney technique”.
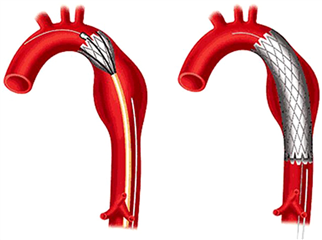


 The TEVAR complications include vascular complications at the puncture site, iatrogenic aortic injury, neurologic complications and endo-leak. The endo-leak is essentially a persistent blood perfusion of excluded aortic pathology. A reason for this may be a leakage at the graft attachment (proximal or distal), a technical issue with the stentgraft or a retrograde filling via a side-branch in the lesion. The first two options are considered severe and require further intervention. Generally is TEVAR less risky in comparison with surgical descending aortic replacement, it has good short- and long-term results. Therefore, it is nowadays a preferred treatment modality of descending aortic aneurysms (in case there are no technical limitations of the procedure).
The TEVAR complications include vascular complications at the puncture site, iatrogenic aortic injury, neurologic complications and endo-leak. The endo-leak is essentially a persistent blood perfusion of excluded aortic pathology. A reason for this may be a leakage at the graft attachment (proximal or distal), a technical issue with the stentgraft or a retrograde filling via a side-branch in the lesion. The first two options are considered severe and require further intervention. Generally is TEVAR less risky in comparison with surgical descending aortic replacement, it has good short- and long-term results. Therefore, it is nowadays a preferred treatment modality of descending aortic aneurysms (in case there are no technical limitations of the procedure).
Acute Aortic Syndrome
Acute aortic syndrome is a newly defined unit, it comprises a group of aortic diseases which require an immediate treatment. To the acute aortic syndromes belong acute aortic dissection, intramural haematoma, penetrating aortic ulcer, pseudoaneurysm of the aorta, aortic rupture and traumatic aortic injury.
ACUTE AORTIC DISSECTION
 It is the most important of the acute aortic syndromes. It is defined as a disruption of media layer of the aortic wall by bleeding. Intima and inner part of media are split from the outer part of media and anventitia. This leads to formation of true and false lumen with or without communication between them. Normally, an intimal tear is found, so called “entry”, from which the blood fills the false lumen and keeps a flow inside. The false lumen may end blindly in the aortic wall, it may open back to the true lumen (so called “re-entry”), or it may disrupt adventitia and lead to an aortic rupture.
It is the most important of the acute aortic syndromes. It is defined as a disruption of media layer of the aortic wall by bleeding. Intima and inner part of media are split from the outer part of media and anventitia. This leads to formation of true and false lumen with or without communication between them. Normally, an intimal tear is found, so called “entry”, from which the blood fills the false lumen and keeps a flow inside. The false lumen may end blindly in the aortic wall, it may open back to the true lumen (so called “re-entry”), or it may disrupt adventitia and lead to an aortic rupture.
We distinguish 2 types of acute aortic dissection according to the Stanford classification:
- Type A aortic dissection - all conditions with an involvement of ascending aorta
- Type B aortic dissection - an involvement of descending aorta only
This classification has particularly practical meaning. It affects the treatment, as well as the prognosis of the patient. Patients with Type A aortic dissection must be operated acutely, while patients with Type B are mostly treated conservatively or with TEVAR. Prognosis of the first group is significantly worse (20% mortality before arrival to hospital, another 25% in-hospital mortality) than of the second group (10% mortality).
Older De Bakey classification is used minimally nowadays (I. type - dissection involving the whole thoracic and abdominal aorta, II. type - involvement of the ascending aorta only, III. type - involvement of the aorta distally from the left subclavian artery).
The aortic dissection may spread to the side branches of the thoracic, as well as abdominal aorta, or it may compress them by a false lumen and lead to various complications - proximal malperfusion (coronary - myocardial ischemia) or distal malpefusion (neurological symptoms, renal failure, mesenteric ischemia, limb ischemia). Other severe complications include cardiac tamponade, acute aortic regurgitation due to tearing of the anulus and valve cusps, congestive heart failure (caused by acute aortic regurgitation, arterial hypertension or myocardial ischemia).
A cause of death in patients with acute aortic dissection is:
- rupture of the outer layer of the false lumen and subsequent bleeding (cardiac tamponade, hemothorax, rarely hemoperitoneum)
- acute aortic regurgitation and heart failure
- ischemic injury (of the heart muscle, brain, kidneys, bowel, limbs)
Etiology
Acute aortic dissection may occur in patients with sclerotic degeneration of the aorta and/or aortic valve. It is frequent in patients with congenital connective tissue disorders (Marfan syndrome and others), after blunt chest injury, in intravenous drug abusers, iatrogenic after cardiac surgery, endovascular procedures, contrapulsation, after coronarography or TAVI. Sometimes it may also affect people with aortic wall of normal quality. The risk factors are arterial hypertension, old age, male sex, smoking.
Symptoms
The symptoms of acute aortic dissection develop quickly. Typical is a sharp tearing pain of the chest or between the shoulder blades, and syncope. Another symptoms are signs of complications:
- hemorrhagic and hypovolemic shock (cardiac tamponade, hemothorax, oliguria, anuria)
- acute myocardial infarction (occlusion of coronary ostia)
- symptoms of an injury to central nervous system (cerebral vessel occlusion)
- paraplegia (impairment of spinal cord blood supply)
- bowel necrosis (mesenteric vessel occlusion)
- lower limb ischemia (occlusion of iliac and femoral vessels)
Diagnosis
History - character and time of pain onset, loss of consciousness, other symptoms of dissection, presence of risk factors, connective tissue disorders, drug abuse
Chest X-ray - pathologic widening of cardiac silhouette and mediastinum, pleural effusion (mostly left-sided)
ECG - possible ischemic changes, left ventricular hypertrophy in arterial hypertension
ECHO - transthoracic, but mostly transoesophageal. It is able to detect and classify the dissection, to assess the aortic valve function, left ventricular ejection fraction and eventual pericardial effusion.
CT - with or without contrast medium. A dominant diagnostic modality. It leads not only to prove the presence of dissection, but also to exactly determine extent of the disease, spreading into side branches, presence of the entry and another aortic pathologies. An advantage is a possibility of 3D reconstruction.
MRI - with or without contrast medium. Considered as a method with the highest imaging quality, however, is not used routinely in acute settings.


Therapy
Acute aortic dissection type A is undoubtedly indicated for urgent surgical treatment. Without the surgery, 90% of patients die in one month. Thanks to surgery it drops to 25-30%. The aim of operation is to re-establish the flow in true lumen and ideally remove the false lumen entry. The aortic valve is seldom destructed, therefore it is mostly justified to perform one of the aortic valve sparing procedures (Yacoub or David). When the dissection continues to the side branches it is required to reconstruct their offsets. The aorta is replaced using some of the abovementioned techniques in accordance with the extent and location of the disease (see aortic aneurysm section). A situation is complicated by the fact that the aortic wall tissue is lacerated and mechanically weakened. As a prevention of suture tearing, one may use pledgets, felt strips, special suture types or tissue glues. Another complicating fact is a failure of coagulation and multi-organ dysfunction arising from the original disease, emphasized by extracorporeal circulation and hypothermia.
Acute aortic dissection type B is primarily treated conservatively. The therapy concentrates on management of pain, heart rate and blood pressure. Patient must keep still in bed and repeated imaging surveillance in the first week after accident is compulsory (ideally CT or MRI).
Intervention is required only in complicated type B dissections. Here belong: uncontrollable or recurrent pain, uncontrollable hypertension despite optimal medical treatment, significant aortic expansion on control imaging, signs of organ malperfusion or aortic rupture (hemothorax). Preferred treatment modality is TEVAR whose aim is to overlap the entry with subsequent improvement of distal perfusion. A stasis of blood in false lumen leads to its thrombosis, remodeling and stabilization. Surgical therapy comes into consideration only when endovascular treatment is contraindicated - severe damage to lower limb arteries, tortuosity of pelvic vessels or aorta, or insufficient proximal landing zone for stentgraft . However, the operations of descending aorta have high mortality (25-50%) and high risk of complications (spinal cord ischemia, stroke, mesenteric or renal ischemia).


INTRAMURAL HAEMATOMA
 Intramural haematoma is defined as a presence of haematoma in the media layer of aortic wall, with no presence of false lumen or intimal tear. There is no blood flow inside. The vascular wall is usually affected in a circular or “crescendo” shape. Etiology, symptoms and classification are analogous with aortic dissection.
Intramural haematoma is defined as a presence of haematoma in the media layer of aortic wall, with no presence of false lumen or intimal tear. There is no blood flow inside. The vascular wall is usually affected in a circular or “crescendo” shape. Etiology, symptoms and classification are analogous with aortic dissection.
The only effective modalities in diagnosis of intramural haematoma are CT and MRI. The second one may be preferred while it offers more accurate differentiation from atherosclerotic lesions, thrombi or thrombosed dissection.
Due to a fact that it has similar prognosis to aortic dissection, the same therapeutic approach is recommended. Intramural haematoma type A is treated surgically (except of the elderly and high risk patients where the conservative treatment may be preferred). Haematoma type B is treated conservatively, an intervention is indicated only in case of complications - analogous with the aortic dissection.
PENETRATING AORTIC ULCER
 Penetrating aortic ulcer is defined as an ulceration of atherosclerotic plaque in aorta that penetrates through internal elastic lamina into the media. By propagating of an ulcer, an intramural haematoma (or an aortic dissection, pseudoaneurysm or aortic rupture) can arise. The underlying pathology is mostly excessive atherosclerosis of the descending thoracic aorta. Risk factors include older age, male sex, smoking, arterial hypertension, coronary artery disease, chronic obstructive pulmonary disease, aneurysm of another aortic region.
Penetrating aortic ulcer is defined as an ulceration of atherosclerotic plaque in aorta that penetrates through internal elastic lamina into the media. By propagating of an ulcer, an intramural haematoma (or an aortic dissection, pseudoaneurysm or aortic rupture) can arise. The underlying pathology is mostly excessive atherosclerosis of the descending thoracic aorta. Risk factors include older age, male sex, smoking, arterial hypertension, coronary artery disease, chronic obstructive pulmonary disease, aneurysm of another aortic region.
Symptoms are analogous with acute aortic dissection. Signs of organ malperfusion occur rarely. Sovereign diagnostic modality is CT.
The aim of therapy is a prevention of aortic rupture and of progression to aortic dissection. Pharmacological treatment is a keystone of patient care. In an ulcer type A the operative treatment is preferred strategy, in type B it is the conservative strategy or TEVAR when complications are present (uncontrollable pain, rapid growing ulcer, periaortic hematoma, pleural effusion).
AORTIC PSEUDOANEURYSM
 It is an aortic dilation due to disruption of all layers of the aortic wall, only the surrounding connective tissue protects it from fatal rupture. As far as intravascular pressure exceeds the maximal tolerated wall tension, the aorta ruptures and patient exsanguinates. The pseudoaneurysm may lead to a formation of fistula or compression of surrounding tissues.
It is an aortic dilation due to disruption of all layers of the aortic wall, only the surrounding connective tissue protects it from fatal rupture. As far as intravascular pressure exceeds the maximal tolerated wall tension, the aorta ruptures and patient exsanguinates. The pseudoaneurysm may lead to a formation of fistula or compression of surrounding tissues.
Pseudoaneurysm arises mostly as a consequence of blunt thoracic injury with rapid deceleration (car accidents, falls, sport injuries). Other causes are aortic infections, penetrating aortic ulcer, iatrogenic injury during interventional or cardiac surgical procedures.
The surgical or endovascular treatment is indicated always after diagnosing this condition.
(CONTAINED) RUPTURE OF AORTIC ANEURYSM
 It should be considered in every patient with detected aortic aneurysm suffering from acute chest pain. A characteristic sign is that bleeding is stabilized by adhesions with surrounding perivascular organs, pleura and pericardium. Therefore, the patient may be hemodynamically stable. When this condition is suspected, a CT scan of the whole torso together with iliac and femoral vascular bed must be performed immediately (for preprocedural planning).
It should be considered in every patient with detected aortic aneurysm suffering from acute chest pain. A characteristic sign is that bleeding is stabilized by adhesions with surrounding perivascular organs, pleura and pericardium. Therefore, the patient may be hemodynamically stable. When this condition is suspected, a CT scan of the whole torso together with iliac and femoral vascular bed must be performed immediately (for preprocedural planning).
This state requires an immediate intervention, the patient is endangered by rapid and massive bleeding in case of rupture. The preferred modality is TEVAR when possible.
Traumatic Aortic Injury
Traumatic injuries of thoracic aorta can be primarily divided into penetrating and blunt. Penetrating injuries (stab or gunshot) are rare and mostly instantly fatal. Blunt aortic injuries are the second most frequent cause of death from non-penetrating trauma right after cerebral injury. 75-90% of patients die at the accident scene. A tear in aortic wall arises due to rapid deceleration in high-speed car accidents and falls from height. Mostly it is located right below the left subclavian artery, rarely in the brachiocephalic trunk region, at the left carotid artery or in the aortic root.
We distinguish 4 degrees of blunt aortic injury according to the following classification:
- degree - an intimal tear
- degree - intramural haematoma, no signs of outer wall layer damage
- degree - pseudoaneurysm
- degree - rupture of the whole aortic wall and exsanguination
Diagnosis
Symptoms are caused by aortic damage together with another injuries in polytrauma. The following signs may be suspicious of blunt aortic injury:
- shock (systolic blood pressure <90 mmHg)
- discrepant blood pressure on the limbs
- fracture of sternal bone, more ribs, shoulder blade and clavicle, impression of steering wheel on the chest
- hemothorax and widened mediastinum on chest X-ray
- heart murmurs
- hoarseness
- dyspnoea
- back pain
- paraplegia or paraparesis
Patient management
The management of polytraumatized patient is led in accordance to ATLS standard (Advanced Trauma Life Support), independently from the suspicion of aortic injury. An examination process includes the primary survey, X-ray of body cavities and limbs where there is a suspicion of fracture, sonographic examination of body cavities, secondary survey.
Hemodynamically unstable patients must be transported to the operating room as soon as possible without further diagnostics to stop the bleeding (mostly in abdominal cavity or brain). To assess the eventual traumatic aortic injury, a transoesophageal echocardiography may be used in the operating room. If an aortic rupture or excess periaortic haematoma is found (4th degree of blunt aortic injury), it must be repaired immediately. In 2nd or 3rd degree of injury, it is more advantageous to postpone the definite aortic reconstruction, to stabilize the patient from the other angles and perform the intervention in 24 hours after trauma.
In hemodynamically stable patient, a CT scan of head and torso with contrast medium according to trauma protocol should be carried out. This examination can reliably detect wall damage or bleeding and assess the degree of injury. MRI and angiography are able to show the aortic wall injury, nevertheless, they are not suitable for acute settings.
The first degree of blunt aortic injury (intimal tear) is treated conservatively. The keystone is an “anti-impulse therapy” which consists in heart rate control by betablockers and blood pressure control (not to exceed 80 mmHg of mean blood pressure). We try to avoid aggressive volumotherapy that may lead to exacerbation of bleeding, coagulopathy and hypertension.
Higher degrees require an intervention in 24 hours after the injury. A trauma of aortic root and ascending aorta is treated by a surgery with extracorporeal circulation. Descending thoracic aortic injuries are mostly treated by implantation of a stentgraft. Surgical therapy comes into consideration only when the TEVAR is not possible, however, it has higher mortality (19% to 9%) and higher risk of paraplegia - it may be reduced using left heart bypass or peripheral extracorporeal circulation.
After handling the aortic lesion and stabilizing the patient, a regular surveillance is recommended. The gold standard for this is CT. Nevertheless, in young people the MRI should be preferred because of lower radiation. The aim of the follow-up is to reveal pseudoaneurysms, endoleak after stentgraft implantation or other complications.


Aortic Tumors
Aortic tumors are extremely rare. Mostly they are sarcomas originating from endothelial cells or from myofibroblasts of intima. Sarcomas of media and adventitia occur minimally. Symptoms are non-specific, usually some mesenteric or peripheral embolizations occur. The disease can imitate gastrointestinal or renal syndromes, spinal disc herniation, peripheral artery disease, or others.
A suitable diagnostic modality for this is MRI of the aorta. In case of positive result, a bone scintigraphy should be carried out due to frequent bone metastases.
The therapy consists in en-block resection of the tumor-involved portion of the aorta and replacement by a tube graft. In most of the times the patient already has metastatic disease at the time of diagnosis, so the surgery is unfeasible. Adjuvant chemo- and radiotherapy can prolong survival. Median survival after diagnosis is 16 months.
Resources:
- Dominik J. Kardiochirurgie. Praha: Grada Publishing, 1998. ISBN: 80-7169-669-2
- Cohn LH, Adam DH. Cardiac Surgery in the Adult, Fifth Edition. New York: McGraw Hill Education, 2016. ISBN 978-0-07184487-1.
- Erbel R, Aboyans V, Boileau C, Bossone E, Di Barolomeo R, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. European Heart Journal 2014; 35: 2873-2926. doi:10.1093/eurheartj/ehu281.
- Di Bartolomeo, Murana G, Di Marco L, Pantaleo A, Alfonsi J, Leone A, et al. Frozen versus conventional elephant trunk technique: application in clinical practice. European Journal of Cardio-Thoracic Surgery 2017; 51: i20-i28. doi:10.1093/ejcts/ezw335
- Dominik J, Žáček P. Chirurgie srdečních chlopní (... nejen pro kardiochirurgy). Praha: Grada Publishing, a.s., 2008. ISBN: 978-80-247-6386-6.
We hereby thank MUDr. Jan Raupach, Ph.D., from the Radiology department of University hospital in Hradec Králové for the cooperation and illustrations for this chapter.
