Pulmonary Embolism
Pulmonary embolism is defined as blockage of the pulmonary vascular bed by an embolism. The most common source of embolism are blood clots from the venous system (mostly from the lower limbs, rarely other locations), it can however be fat embolism, air, embolism of amnionic fluid and other rare cases (septic embolism, tumorous tissues, foreign bodies- catheters a others). The pulmonary vascular bed can be damaged on different levels from the small arteriols to the pulmonary trunk. That explains the wide range of symptoms from asymptomatic to life-threatening obstructive shock.
Pulmonary embolism is the third most common cause of death after heart disease and cancer. In the clinical practice we often see underdiagnosis combined with inadequate therapy which leads to a death rate of about 30% of patients.
Etiology and Pathogenesis
The most common cause of embolism is a deep vein venous thrombus from the lower limbs (80-90% of cases), often from the veins of the ileofemoral area or the pelvic plexus. Sometimes the embolism may originate from the upper limbs or the right heart. Venous thrombosis can be provoked by:
- Orthopedic operations- hip or knee replacement
- Oncological surgical procedures
- Other major surgery (though the risk is lower compared to the above types of surgeries)
- Tumors
- Extensive trauma
- Fracture of the lower limbs
- Other causes of immobility
- Pregnancy
- The use of hormonal contraceptives
Risk factors which can contribute to the formation of thrombus include advanced age, a history of thromboembolism, congenital or acquired hypercoagulable states (deficiency of protein C, protein S, antithrombin III, Leiden mutation of factor V, antiphospholipid syndrome and others)
Pulmonary embolism has severe hemodynamic and respiratory consequences. The hemodynamic consequences depend on the extent of the obstruction of the pulmonary vascular bed. Pulmonary hypertension occurs if the occlusion is more than 30-50%. This state is worsened by humoral reaction (thromboxane A2 and serotonin) which cause vasoconstriction and as such increase pulmonary vascular resistance. The sudden rise in pressure in the pulmonary artery leads to dilation of the right ventricle and a decrease in its contractility. Breathlessness and other signs of right heart failure develop. The interventricular septum deviates to the left and a right bundle branch block can occur on ECG. This may negatively affect the filling of the left ventricle, decreasing cardiac output and finally leading to systemic hypotension and shock.
Respiratory consequences of pulmonary embolism occur on the basis of ventilation/perfusion disbalance of the lung in favor of ventilation. This leads to hypoxemia. Additional mechanisms include shunting of deoxygenized blood from the lung to the systemic circulation across a persistent foramen ovale (in about a third of patients) as a result of high pressure in the right heart. The compensatory mechanism of hyperventilation does not improve hypoxemia but leads to hypocapnia. Small distal embolism can lead to lung infarction which manifests as hemoptysis, pain and breathlessness.
Diagnosis
HISTORY AND PATIENT EXAMINATION
Patients often present with breathlessness, pain (which is dependent on breathing and similar to stenocardia) and cough. Less often hemoptysis, fever or syncope can occur.
In physical examination, symptoms of shock (hypotension, tachycardia) together with increased pulmonary filling can be observed in high risk patients. Almost always there is tachypnoea, crackles on auscultation and an accented second heart sound above the pulmonary artery.
LABORATORY TESTING
D-dimer level testing is the most important. Physiological levels almost always exclude the diagnosis of pulmonary embolism. However, a positive result does not necessarily mean a confirmation of the diagnosis. Blood gases should also be obtained to reveal hypoxemia and hypocapnia. The extent of heart damage can be determined using BNP titers (right heart failure) and cardiac troponins (damage to the myocardium during pulmonary hypertension, in a state of high catecholamine levels and shock).
ECG
It is important to rule out acute myocardial infarct and other patologies in terms of differential diagnosis. Signs of pulmonary embolism on the ECG are of low specificity and include:
- Right Bundle Branch Block
- Right heart strain (negative T-waves in V1-V3, P pulmonale)
- McGinn-White sign- S1Q3T3 (a finding of S-wave in lead 1, Q-wave and negative T-wave in lead 3)
CHEST X-RAY
Dilation of the right heart chambers and the pulmonary artery. Elevated diaphragm, pleural effusion, poor vascularization in the affected area and congestion in the remaining areas. This is however not specific to pulmonary embolism only.
ULTRASOUND

 Transthoracic echography is one of the most important diagnostic modalitites. It is most important in unstable patients at-risk, where it shows dilatation and dysfunction of the right ventricle, deviation of the interventricular septum to the left, tricuspid insufficiency and pulmonary hypertension, dilation of the right atrium and the inferior caval vein. It is also important in the differential diagnosis, it helps to exclude acute myocardial infarction, cardiac tamponade, acute aortic dissection or valvular dysfunction. Sometimes it can even detect thrombi in the right heart chambers. When using trans-oesophageal echocardiography, large branches of the pulmonary vascular bed can be easily visualized.
Transthoracic echography is one of the most important diagnostic modalitites. It is most important in unstable patients at-risk, where it shows dilatation and dysfunction of the right ventricle, deviation of the interventricular septum to the left, tricuspid insufficiency and pulmonary hypertension, dilation of the right atrium and the inferior caval vein. It is also important in the differential diagnosis, it helps to exclude acute myocardial infarction, cardiac tamponade, acute aortic dissection or valvular dysfunction. Sometimes it can even detect thrombi in the right heart chambers. When using trans-oesophageal echocardiography, large branches of the pulmonary vascular bed can be easily visualized.
Sonography of the veins of the lower limbs is of high importance in the diagnosis and subsequent therapy of PE. If thrombosis is found in the proximal veins, no further investigation is necessary and anticoagulant therapy can be initiated. This method is particularly important in patients in whom CT angiography is contraindicated (e.g. in pregnant women). Unfortunately, a negative finding does not rule out the diagnosis of pulmonary embolism and further investigation is required.
CT ANGIOGRAPHY

 The most important diagnostic modality in PE. Requires the administration of contrast media. It is able to produce sufficient image of the pulmonary vascular bed all the way to the segmental level. A finding of thrombus on CT-angiography is sufficient proof to initiate therapy. At the same time, it is used for differential diagnosis of other acute conditions (acute aortic dissection, pneumothorax and others).
The most important diagnostic modality in PE. Requires the administration of contrast media. It is able to produce sufficient image of the pulmonary vascular bed all the way to the segmental level. A finding of thrombus on CT-angiography is sufficient proof to initiate therapy. At the same time, it is used for differential diagnosis of other acute conditions (acute aortic dissection, pneumothorax and others).
LUNG SCINTIGRAPHY
 This is a ventilation-perfusion scan of the lungs using labeled albumin to display blood flow and a labeled aerosol to display ventilation. Evidence of PE is a mismatch between ventilation and perfusion in favor of ventilation. This method can be used instead of CT-angiography because of lower radiation load in younger patients, in pregnant women, as well as in patients with contraindications to the use of a contrast medium (allergy, renal failure). Unfortunately, the informative value is not the same as with CT.
This is a ventilation-perfusion scan of the lungs using labeled albumin to display blood flow and a labeled aerosol to display ventilation. Evidence of PE is a mismatch between ventilation and perfusion in favor of ventilation. This method can be used instead of CT-angiography because of lower radiation load in younger patients, in pregnant women, as well as in patients with contraindications to the use of a contrast medium (allergy, renal failure). Unfortunately, the informative value is not the same as with CT.
Other diagnostic modalities are of marginal importance - conventional pulmonary angiography is only appropriate in patients undergoing catheterization for suspected acute coronary syndrome. With this method, it is possible to visualize the pulmonary vascular bed to find defects in vessel filling or flow interruptions. Magnetic resonance imaging is not suitable for the diagnosis of pulmonary embolism.
DIAGNOSTIC STRATEGY
In terms of diagnosis and eventual treatment of patients, it is important to divide patients with pulmonary embolism into two groups:
- High risk acute pulmonary embolism with a high risk of death
- Non-high risk acute pulmonary embolism with a low risk of death
High risk patients are in shock and require immediate therapy. The most appropriate diagnostic modality is a bed-side transthoracic echocardiography. If signs of a right heart failure are present, it is justified to begin a therapy. It is also important to perform an ultrasound of the venous system of the lower limbs. As soon as the patient is stabilized, a CT angiography can be performed.
Non-high risk patients (or hemodynamically stable) should undergo a CT angiography, provided there is a high clinical suspicion of pulmonary embolism (multiple risk factors; there are various scoring systems for this, e.g. Wells, Geneva score). In patients with a low clinical suspicion, a D-dimer level should be checked. If negative, pulmonary embolism can be ruled out. In the case of a positive result, CT should be performed. When attempting to avoid radiation exposure, venous sonography of the lower limbs or lung scintigraphy is appropriate in the above groups.
Therapy
THERAPY OF HIGH-RISK PATIENT
The most important part of management is aggressive antithrombotic therapy. Continuous anticoagulation with unfractionated heparin and systemic thrombolysis is the gold standard of therapy. Because of the high risk of bleeding with systemic thrombolysis (especially intracranial haemorrhage), alternative catheter therapeutic modalities were developed:
- mechanical: catheter embolectomy, catheter thrombus fragmentation
- pharmacological: local administration of thrombolysis via catheter into the pulmonary vascular bed (proven lower risk of bleeding)
- pharmacomechanical thrombolysis (combination of local administration of thrombolysis and ultrasound energy)
Surgical embolectomy is only justified when thrombolysis is contraindicated or failed due to surgical risk. A specific indication is the finding of thrombus in the right chambers of the heart, especially "stuck" in the foramen ovale (there is also a risk of embolization into the systemic circulation). Surgery is performed through median sternotomy in normothermia using extracorporeal circulation.
Mechanical circulatory support is another alternative therapy. By introducing cannulas of the system through the peripheral (femoral) blood vessels, circulation is maintained while the pulmonary vascular system is blocked. It serves as a bridge to transport of patient to the operating room, or it can be used as a solo therapy - the support is driven until the thrombus dissolves and the patient’s own circulation is reestablished.
Supportive therapy is essential and includes artificial pulmonary ventilation, administration of mild amounts of fluids, catecholamines, vasodilators (NO, levosimendan), oxygen therapy, analgotherapy, acid-base management. After stabilizing the patient, an early initiation of oral warfarin anticoagulation is required (unfractionated heparin is administered until an effective dose is titrated).
THERAPY OF NON-HIGH RISK PATIENT
In this low risk group, therapy is limited to anticoagulation with low molecular weight heparin or fondaparinux. At the same time, titration of oral vitamin K antagonist, warfarin, is initiated. Upon reaching an effective INR (2-3), heparin is discontinued, and oral therapy is continued only. Alternatives to warfarin such as the new oral anticoagulants (NOAC), i.e. dabigatran, rivaroxaban, apixaban, edoxaban can also be used. The degree of anticoagulation and the reduction of the risk of further thromboembolic events are comparable, while the risk of bleeding complications is significantly lower compared to warfarin. Immobilization of PE patients is not necessary. Anticoagulation therapy lasts for at least 3-6 months (as a secondary prevention of thrombembolism) and its further continuation should be considered based on individual risk factors.
In patients in whom anticoagulant therapy is contraindicated, or in recurrent thromboembolism despite effective anticoagulation, the introduction of a caval filter (into the lower caval vein) is required, which prevents further embolization. The effectiveness in preventing PE is high, but it carries the risk of filter migration, destruction and embolization of foreign particles, or penetration of the vena cava inferior.
PRIMARY PREVENTION OF THROMBEMBOLISM
In the surgical setting, primary prevention of thromboembolism is of great importance. Patients undergoing hip or knee replacement are at the highest risk, followed by patients after oncological surgery. However, any other major surgical procedures associated with immobilization also carry a significant risk of deep vein thrombosis and pulmonary embolism.
Non-pharmacological methods include early mobilization of the patient after surgery. Complementary tools are compression leg bandages, elastic stockings or intermittent pneumatic compression. Rarely it is justified to use a caval filter as a primary prevention (in trauma patients).
Pharmacological prevention is represented by administration of a preventive dose of low molecular weight heparin or fondaparinux during hospitalization. After orthopedic procedures, NOAC administration is advisable. Warfarin has no place in the prevention of early postoperative thrombosis; it is however indicated when prolonged prophylaxis is required in a subacute or chronic regime (e.g. after extensive trauma).
Other causes of pulmonary of embolism
Septic embolization most often accompanies right-sided infectious endocarditis. The evidence is based on the finding of an infective focus (detection of vegetation on the tricuspid valve or on pacemaker electrodes), positive blood cultures and a typical finding on chest X-ray or CT (nodular foci with decay process). Therapy of the underlying disease (antimicrobial therapy, removal of infective focus) is a priority here.
Fat embolism occurs after large bone trauma or orthopedic surgery, bone marrow transplantation, liposuction or acute pancreatitis. The manifestations are not only associated with vascular obstruction by lipid particles, but also with a subsequent inflammatory reaction. Lung, brain and skin mircocirculation is impaired, and this is associated with the typical trias of symptoms: impaired consciousness, respiratory failure and petechiae on the skin. Patient care is based on supportive symptomatic therapy, corticoids and albumin, which improve prognosis.
Air embolism arises as an iatrogenic complication of manipulation of central venous and hemodialysis catheters, neurosurgical operations in the sitting position and injuries. The air together with fibrin clog the outflow tract of the right ventricle or pulmonary arterioles, leading to hemodynamic decompensation. The therapy is an attempt for air aspiration and oxygen therapy (air can be absorbed by diffusion).
Amniotic fluid embolism occurs in severe labor, in stillbirth, placental disruption by trauma or surgery. The amniotic fluid reaches the uterine veins and the pulmonary vascular bed causing obstruction by the clumping of cells and meconium. An inflammatory reaction often follows the obstruction. The patient develops pulmonary edema and acute respiratory distress. The therapy is symptomatic.
Embolization of foreign bodies - most often embolization of catheter fragments, cannulae, caval filters, endovasal stents. The ideal solution is removal by catheterization.
Embolization of tumor cells occurs most frequently in renal tumors with invasion into the venous system, rarely also in tumors of the prostate, gastrointestinal tract, liver and breast. The clinical presentation mimics various lung diseases (pneumonia, tuberculosis, interstitial disease), in macroembolizations it is indistinguishable from venous thrombembolism. Therapy consists mainly in causal therapy of the tumor.
Chronic thrombembolic pulmonary hypertension
Chronic thrombembolic pulmonary hypertension is a specific chronic disease that is a late complication of pulmonary embolism, especially repeated pulmonary embolisations. It is worth mentioning here because cardiac surgery is the treatment of choice.
Pathogenesis. Pulmonary embolism, sometimes reccurent, leads to consecutive obstruction of the pulmonary vascular bed. This is followed by humoral reactions, gradual pulmonary vascular remodeling and secondary in situ thrombosis. Pulmonary microvasculopathy develops and pulmonary vascular resistance increases. Inadequate anticoagulation, large mass of thrombus, infection, oncological disease, acquired hypercoagulable state or autoimmune disease may also play a role in this.
The diagnosis is based on clinical suspicion of the disease. It is problematic because it has no specific symptom. In the foreground there are dyspnoea, non-specific chest pain, sometimes edema and hemoptysis. The important step in the diagnosis is echocardiography, which shows the involvement of the right-sided chambers, dominated by tricuspid regurgitation and pulmonary hypertension. In case of a positive finding, ventilation - perfusion lung scintigraphy is performed. Conventional pulmonary angiography, which is the gold standard of evidence and assessment of the extent of the disease, is mandatory when disease is confirmed. An atypical contrast filling defect, strips, or complete contrast flow obstruction are seen therein.

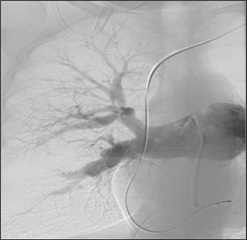
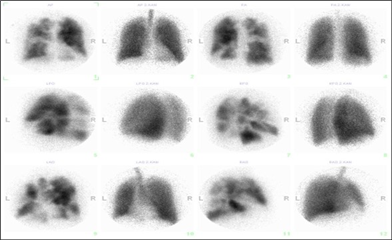
Therapy. Chronic anticoagulant therapy with warfarin with a target INR of 2-3 is indicated. It may lead to a slight improvement in symptoms. Right-sided failure is treated with diuretics and vasodilators, but is only considered as supportive therapy. If effective anticoagulation for 3 months does not lead to improvement, surgical therapy should be considered. The causal and ultimate curative therapeutic modality is surgical endarterectomy of the pulmonary artery. The procedure is performed through median sternotomy approach with extracorporeal circulation, in deep hypothermic circulatory arrest. Circulatory arrest is necessary due to the numerous collaterals between the pulmonary and systemic circulation, with the aim of accurately removing the affected pulmonary endarterium and its branches in bloodless surgical field. Thus, it is not only a simple thrombectomy (removal of the thrombus only), but a true endarterectomy (the thrombus is removed along with the inner layer of the vessel wall). Surgery leads to a symptomatic and objective improvement of the patient's condition, pulmonary vascular resistance decreases and right heart function returns to normal. The lifelong anticoagulation is obligatory. In the case of persistent pulmonary hypertension, even after successful surgery, specific vasodilators (riociguat) may be considered.
Regarding localization of pulmonary vascular bed involvement we distinguish 4 types of lesions:
 type - central thrombi
type - central thrombi- type - thrombi in segmental branches
- type - thrombi in subsegmental branches
- type - peripheral lesion and thrombi that arise in situ during pulmonary arterial hypertension
The surgery has the greatest benefit in lesions affecting the pulmonary artery and its large branches (type 1-2 according to the abovementioned classification). The third type has a borderline indication for surgical intervention, the benefit is small to uncertain, but technically still feasible. If only distal branches (type 4) are affected, the indication is questionable, and patients do not benefit from surgery. The results of pulmonary endarterectomy are generally very satisfactory, surgical mortality is below 5%, some centres report even below 2%, long-term results are favorable.
Catheterization therapy - balloon pulmonary angioplasty could be a modern alternative to endarterectomy, especially in inoperable patients. However, its benefits currently have not reached the level of surgical therapy and repeated sessions are required.
Resources:
- Klener P, et al. Vnitřní lékařství - Čtvrté, přepracované a doplněné vydání. Praha: Galén, 2015. ISBN: 978-80-7262-705-9.
- Češka R a kol. Interna. Praha: Triton, 2010. ISBN: 978-80-7387-423-0.
- Cohn LH, Adam DH. Cardiac Surgery in the Adult, Fifth Edition. New York: McGraw Hill Education, 2016. ISBN 978-0-07184487-1.
- Konstantinides V, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal 2014; 35: 3033-3080. doi:10.1093/eurheartj/ehu283.
- Konstantinides SV, Barco S, Lankeit M, Meyer G. Management of Pulmonary Embolism, An Update. Journal of the American College of Cardiology 2016; 67(8): 976-90. doi: http://dx.doi.org/10.1016/j.jacc.2015.11.061.
- Dudzinski DM, Giri J, Rosenfield K. Interventional Treatment of Pulmonary Embolism. Advances in Interventional Cardiology 2017; 10:e004345. doi: 10.1161/CIRCINTERVENTIONS.116004345.
- Lang I, Meyer BC, Ogo T, Matsubara H, Kurzyna M, Ghofrani HA et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. European Respiratory Review 2017; 26: 160119. doi: https://doi.org/10.1183/16000617.0119-2016.
We hereby thank MUDr. Matúš Nižňanský from General university hospital in Prague for consulting and illustrations in CTEPH chapter.
