Mitral & Tricuspid Valve Disease
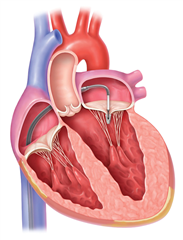
Mitral and tricuspid valves, together as atrioventricular valves, are placed between atria and ventricles of the heart. Their role is to prevent retrograde blood flow from ventricles to atria in systole and to enable resistance-free diastolic filling of ventricles. Their function can be impaired in terms of stenosis or regurgitation, leading to heart failure, atrial fibrillation and thrombus formation.
Mitral Valve Disease - Etiology and Pathogenesis
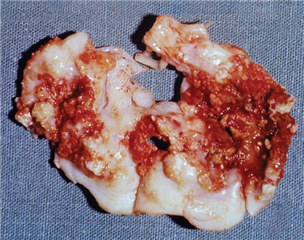 Mitral valve stenosis is mostly of rheumatic etiology. Years after rheumatic fever, valve leaflets start to form adhesions in commissures and to fibrotically thicken due to autoimmune processes. Simultaneously the tendinous chords get affected in terms of shortening and thickening. The diastolic filling of the left ventricle is impaired what leads to volume- and pressure-overload of the left atrium. This is expressed as its hypertrophy and dilation. The blood stagnates in pulmonary circulation and the post-capillary pulmonary hypertension arises. In late stage this can lead to secondary tricuspid valve regurgitation. Dilation of the left atrium is a predisposition for arrhythmias, most of all the atrial fibrillation, and for subsequent formation of thrombi and systemic embolization. Combining these mechanisms leads to decompensation - heart failure and pulmonary edema.
Mitral valve stenosis is mostly of rheumatic etiology. Years after rheumatic fever, valve leaflets start to form adhesions in commissures and to fibrotically thicken due to autoimmune processes. Simultaneously the tendinous chords get affected in terms of shortening and thickening. The diastolic filling of the left ventricle is impaired what leads to volume- and pressure-overload of the left atrium. This is expressed as its hypertrophy and dilation. The blood stagnates in pulmonary circulation and the post-capillary pulmonary hypertension arises. In late stage this can lead to secondary tricuspid valve regurgitation. Dilation of the left atrium is a predisposition for arrhythmias, most of all the atrial fibrillation, and for subsequent formation of thrombi and systemic embolization. Combining these mechanisms leads to decompensation - heart failure and pulmonary edema.
The incidence of rheumatic mitral valve disease is nowadays significantly lower thanks to eradication of rheumatic fever and effective antibiotic treatment of streptococcal infections. Mitral valve stenosis is the most common form of rheumatic heart disease, rarely it occurs together with aortic valve disease. Isolated aortic valve disease or pancarditis are extremely scarce. Other causes of stenosis are carcinoid, heart tumors, big vegetations, extensive calcifications of the valve in elderly patients, congenital valve defects or congenital metabolic disorders (mucopolysaccharidoses).
Mitral regurgitation is basically a retrograde flow from the left ventricle to the atrium in systole. Apart from the standard prograde stroke volume, the left ventricle has to pump also the regurgitant fraction of the blood, which pendulates between the atrium and the ventricle. To preserve adequate tissue perfusion the cardiac output must increase. This leads to volume-overload of the left ventricle and its excentric hypertrophy. The left atrium dilates, as well. That predisposes to the atrial fibrillation and systemic embolization. After exhausting the compensatory mechanisms (or in acute settings), left heart failure occurs together with pulmonary hypertension, secondary tricuspid regurgitation and pulmonary edema.
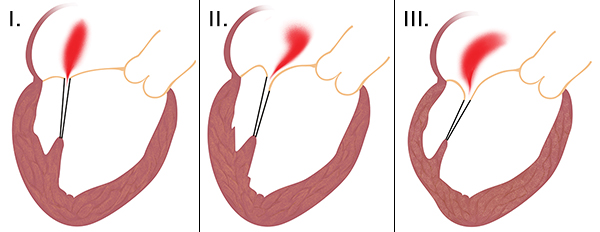
The mechanisms of mitral valve regurgitation can be divided in accordance to Carpentier classification (this is particularly important for choosing of surgical strategy):
- type - dilation of mitral valve anulus and subsequent insufficient leaflet coaptation. The leaflets alone are not diseased.
- type - leaflet prolapse above the level of the mitral anulus. It is caused by excess myxomatous leaflet tissue or disease of subvalvular apparatus (chordae and papillary muscles).
- type - restrictive leaflet motion. The leaflet does not reach the level of the mitral anulus, mostly in disease of subvalvular apparatus or ventricle itself.
The causes of mitral regurgitation can be divided into primary and secondary. Primary (organic) disease affects the valve itself and its subvalvular apparatus:
- myxomatous degeneration (Morbus Barlow) - most common form, characterized by dilation and deformation of the anulus, excess leaflet tissue, formation of calcifications, thickening and elongation of tendinous chords
- fibroelastic degeneration - typical is attenuation, elongation and rupture of chords
- rheumatic disease - thickening of the valve leaflets and subvalvular apparatus together with adhesions, leading to leaflet malcoaptation
- infective endocarditis - infection can lead to rupture or perforation of the leaflets and subvalvular apparatus, vegetation can be often found on the atrial side (bringing the risk of systemic embolization)
- papillary muscle rupture (mechanical complication of acute myocardial infarction)
- congenital defect (anterior leaflet fissure in atrioventricular septal defect)
- congenital tissue disorders (Marfan and Ehlers-Danlos syndrome)
- radiation, drugs, trauma, iatrogenic lesions, other rare causes...
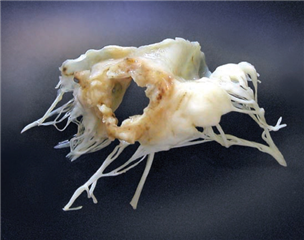
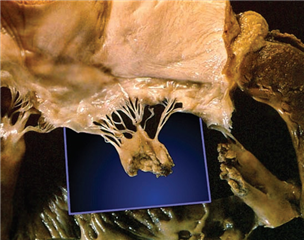
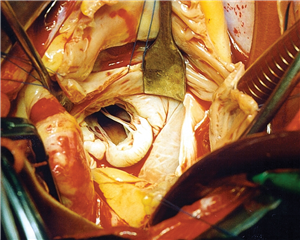
In secondary (functional) mitral valve regurgitation the valve and the subvalvular apparatus are intact, it is a disease of the left ventricle - there is an impaired relationship between the left ventricle and the mitral valve. It is described as dilation of the mitral valve anulus and retraction of the valve leaflets via the subvalvular apparatus to the ventricle, that is remodelled and dilated. The cause of this is chronic ischemic disease of the left ventricle or dilated cardiomyopathy.
The surgery for mitral valve regurgitation is the second most common cardiac valve procedure in adults in modern society, right after the aortic valve replacement.
Mitral Valve Disease - Diagnosis
SYMPTOMS
Stenosis, as well as regurgitation, manifest as exertional dyspnea, exhaustion, moreover, in atrial fibrillation as palpitations and systemic embolizations. Cough and hemoptysis may be seen in stenosis. Acute mitral regurgitation (e.g. in infective endocarditis or ischemic papillary muscle rupture) is manifested as acute left heart failure and pulmonary edema.
Dyspnea on exertion can be divided into 4 functional classes according to popular NYHA classification (New York Heart Association):
- No limitations in activities of daily living. Difficulties only with extreme or prolonged workload.
- Mild limitation in activities of daily living. Difficulties during increased workload - >1 floor upstairs, uphill walking, fast walking on levelled ground.
- Significant limitation in activities of daily living. Difficulties during light workload - normal pace walking on levelled ground, <1 floor upstairs, dressing up, shower.
- Difficulties at rest.
PHYSICAL EXAMINATION
Stenosis is characterized by a diastolic murmur above the cardiac apex, sometimes the opening snap can be heard. „Facies mitralis“ can be seen (pink-to-violet cheek color and venectasiae in contrast with pale face caused by low cardiac output).
Regurgitation is characterized by systolic murmur above the cardiac apex propagating to the left armpit. Sometimes the third heart sound can be noticed.
ECG
Non-typical. P mitrale can be present (prolonged and biphasic P wave due to hypertrophy of left atrial myocardium). In late stage the atrial fibrillation occurs.
CHEST X-RAY
Mitral shape of the heart is typical for the stenosis - straightened left contour because of left atrial and right ventricular dilation. The signs of pulmonary congestion (interstitial pulmonary edema, Kerley lines, alveolar pulmonary edema).
ECHOCARDIOGRAPHY
Gold standard in mitral disease evaluation. It serves to assess presence, significance, etiology, mechanism of the valve disease and consequences (dilation and hypertrophy of heart chambers, reduction in ejection fraction of the left ventricle, presence and severity of the pulmonary hypertension, eventual secondary tricuspid regurgitation). First step in the diagnostics is the transthoracic ECHO (TTE), in case of uncertain finding the transoesophageal ECHO (TOE) is applicable. TOE is currently able to produce also 3D reconstructions (including „surgical view“, that imitates the view of the valve through the opened left atrium). A flawless echocardiographic examination including the assessment of disease mechanism is essential for choosing of the correct surgical treatment strategy.
Sever mitral valve stenosis has these ECHO attributes:
- calcification and thickening of the valve leaflets and subvalvular apparatus, impairment of the valve motion
- mitral valve orifice area <1.5 cm2
- mitral valve orifice area indexed on the body surface area <0.8 cm2/m2
- mean diastolic gradient >8 mmHg
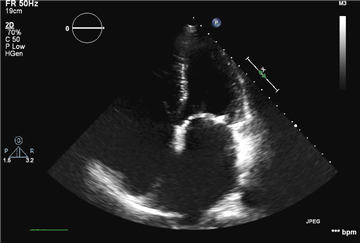
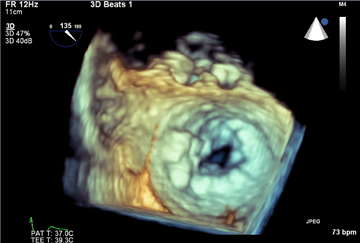
Severe mitral valve regurgitation is characterized by these ECHO signs:
- visible leaflet prolapse, rupture of the papillary muscle, severe coaptation defect
- severe central / excentric regurgitation jet reaching the back wall of the left atrium is to see on Doppler imaging
- vena contracta >7 mm
- systolic blood flow reversal in pulmonary veins
- E wave dominance in transmitral blood flow in systole
- regurgitation orifice area >40 mm2 in primary and >20 mm2 in secondary valve disease
- regurgitation volume >60 ml in primary and >30 ml in secondary valve disease
- dilation of the left atrium and the left ventricle
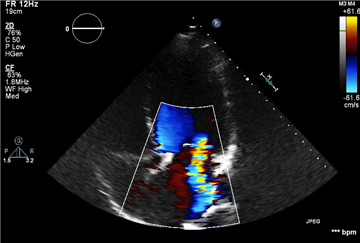
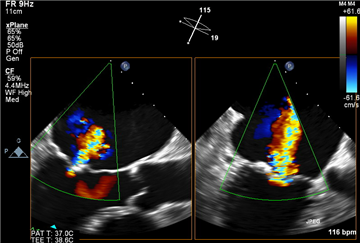
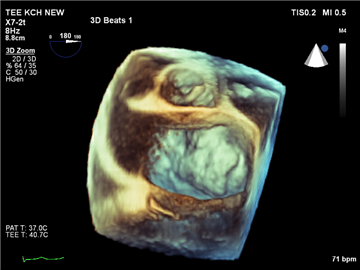
Stress echocardiography plays a role in revealing the symptoms in „asymptomatic“ patients, and, apart from that, it may detect significant post-exertional changes in transmitral gradient or in pulmonary pressure. That could lead to earlier surgical intervention.
HEART CATHETERISATION
Invasive diagnostic modality, contrast imaging of the left ventricle (ventriculography), with visualization of the regurgitation jet and measurement of gradients in heart chambers used to be the gold standard in mitral valve disease evaluation. Currently it retires in favor of echocardiography, it plays a role in coronary vascular bed examination for eventual concomitant surgical myocardial revascularization.
MAGNETIC RESONANCE IMAGING
According to the newest data it is the most accurate modality to quantify the mitral regurgitation. However, it is not carried out routinely.
Mitral Valve Disease - Therapy
Conservative treatment is similar in both types of the valve disease. It concentrates on 3 problems:
- prevention of infective endocarditis (antibiotic prophylaxis during dental procedures etc.)
- atrial fibrillation treatment (antiarrhythmic drugs, electrocardioversion, anticoagulation)
- heart failure treatment (diuretics, ACE inhibitors etc.)
SURGICAL TREATMENT OF MITRAL STENOSIS
It is indicated in symptomatic patients with severe mitral valve stenosis according to ECHO. Exceptionally it is possible to proceed to the surgery in a non-severe stenosis, where there is no other explanation for symptoms. Asymptomatic patients are indicated for surgery in case of high risk for systemic embolization (e.g. in atrial fibrillation) or for hemodynamic decompensation (high pulmonary pressure, desire for pregnancy, concomitant cardiac surgery).
There is an excellent interventional treatment method - balloon valvuloplasty, which is suitable for some patients with severe mitral valve stenosis. They may have adhesions in commissures, but the valve must not be calcified, regurgitant and the subvalvular apparatus must not be affected. ECHO criteria are often not fulfilled in our population because of severe valve disease, therefore the surgery is mostly preferred. Surgery consists in valve replacement in most of the times, rarely is it possible to preserve the valve and to perform the commissurotomy only or other valve adjustment.
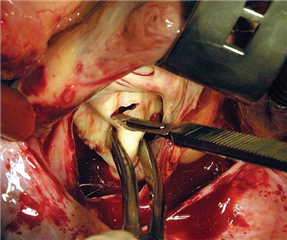
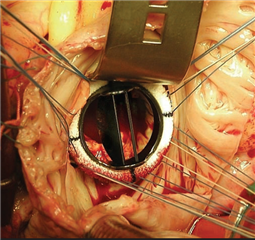 Mitral valve replacement is considered gold standard in the treatment of excessive stenotic mitral valve disease. Mechanical and biological prostheses are at disposal, the choice is in accordance to age, lifestyle and patient comorbidities. The topic is discussed in detail in the chapter of aortic valve disease. The only important difference is that the age limit for biological prosthesis implantation is 70-75 years because of its stronger tendency to degenerate - below this value the mechanical prostheses are being implanted.
Mitral valve replacement is considered gold standard in the treatment of excessive stenotic mitral valve disease. Mechanical and biological prostheses are at disposal, the choice is in accordance to age, lifestyle and patient comorbidities. The topic is discussed in detail in the chapter of aortic valve disease. The only important difference is that the age limit for biological prosthesis implantation is 70-75 years because of its stronger tendency to degenerate - below this value the mechanical prostheses are being implanted.
The approach to the valve is median sternotomy (or minimally invasive approach of right anterolateral thoracotomy). The extracorporeal circulation is introduced, heart is stopped and the surgeon approaches the valve mostly from behind through the interatrial groove, or through the right atrium and interatrial septum. In contrast to aortic valve replacement, it is beneficial to leave anterior leaflet of the mitral valve in situ, in order to preserve continuity of mitral anulus, valve leaflet, subvalvular apparatus and free ventricular wall. To keep this architecture it is allowed to preserve only a part of the anterior leaflet, i.e. its subvalvular apparatus. Thanks to these techniques it is possible to minimize the feared postoperative left ventricular dilation and drop of the ejection fraction.
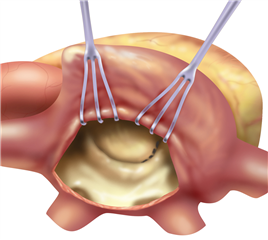
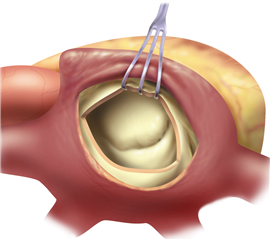
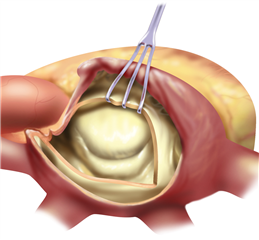
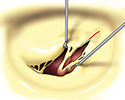 The valve-sparing procedures of the mitral valve (plasties) are currently rare in stenosis. The basic procedure is the open mitral commissurotomy on stopped heart - the surgeon performs an incision through the commissural adhesions, that leads to improvement in valve opening. Sometimes it is required to cut the chordea (and the papillary muscles) in half, or proceed to more complicated steps as resection of thickened leaflet tissue, partial leaflet replacement with autologous pericardium etc.
The valve-sparing procedures of the mitral valve (plasties) are currently rare in stenosis. The basic procedure is the open mitral commissurotomy on stopped heart - the surgeon performs an incision through the commissural adhesions, that leads to improvement in valve opening. Sometimes it is required to cut the chordea (and the papillary muscles) in half, or proceed to more complicated steps as resection of thickened leaflet tissue, partial leaflet replacement with autologous pericardium etc.
SURGICAL TREATMENT OF MITRAL REGURGITATION
Primary mitral regurgitation is indicated for surgery in symptomatic patients with severe mitral valve regurgitation according to ECHO. Asymptomatic patients are indicated in case of drop in left ventricular ejection fraction below 60%, the left ventricle is dilated in systole (>45 mm), the atrial fibrillation or pulmonary hypertension (>50 mmHg) are present. With regard to excellent short- and long-term outcomes of mitral valve plasty with low mortality, it is sometimes possible to proceed to surgery in asymptomatic patients without these characteristics (in case of high success probability). The indication is controversial in patients with severe left ventricular dysfunction (ejection fraction <30%) and in patients with severe comorbidities and high risk of surgery.
Secondary mitral valve regurgitation is a debatable indication for surgery. It is essentially not the disease of the valve itself, but the disease of myocardium. The outcomes are characterized by worse prognosis in comparison with surgery for primary mitral regurgitation. It has not been definitely proved, that the surgery improves patient survival. Surgery of secondary mitral valve regurgitation is justified in patients with severe valve disease undergoing concomitant coronary artery bypass grafting.
The therapy of choice in mitral valve regurgitation is the valve-sparing procedure - mitral valve plasty. In contrast to mitral valve replacement it is associated with significantly lower morbidity and mortality. Patients are not limited by lifelong anticoagulation, therefore, there is no risk of bleeding or thrombembolism. The risk of re-do surgery is low. Particularly in degenerative mitral valve regurgitation it is possible to perform a successful plasty in 95% of patients. One of the greatest pioneers of this technique, Alain Carpentier, summarized the principles of mitral reconstructive surgery into 3 golden rules:
- preserve leaflet motion
- restore sufficient leaflet coaptation surface
- stabilize the mitral valve anulus
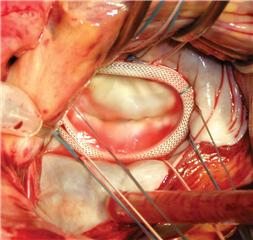 An integral part of practically each mitral valve plasty is the stabilization of anulus using the anuloplasty ring. There are various technical options (complete or incomplete ring, rigid, semi-rigid or flexible ring etc.). The aims of ring implantation are to fix the mitral valve anulus in the right shape and to adjust its diameter to correct size. In case when the anulus dilation is the only mechanism of regurgitation, this technique is sufficient to completely restore the valve function.
An integral part of practically each mitral valve plasty is the stabilization of anulus using the anuloplasty ring. There are various technical options (complete or incomplete ring, rigid, semi-rigid or flexible ring etc.). The aims of ring implantation are to fix the mitral valve anulus in the right shape and to adjust its diameter to correct size. In case when the anulus dilation is the only mechanism of regurgitation, this technique is sufficient to completely restore the valve function.
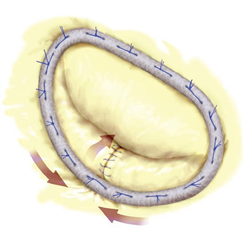
Apart from the anuloplasty, it is possible to operate on the valve leaflets and subvalvular apparatus. On the posterior leaflet, quadrangular or triangular resection of prolapsed segment may be performed. In case of excess tissue, a more radical procedure - sliding plasty - may be performed together with quadrangular resection. On the anterior leaflet, one has to be more careful in resection procedures, with the aim to preserve the leaflet anatomy (the closeness of both fibrous trigones and of aortic valve). The resections of arterior leaflet are restricted to slight triangular resection.
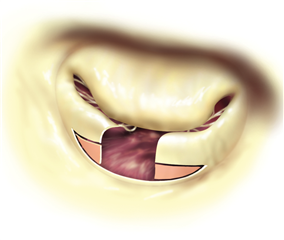
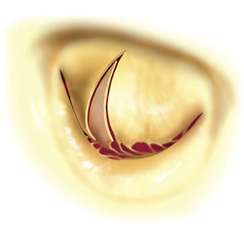
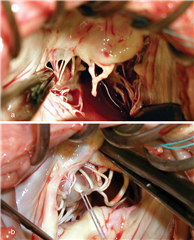
 In cusp prolapse it is possible to operate on subvalvular apparatus, as well. Chordal shortening, or replacement by artificial goretex chords provide retraction of the prolapsed segments into the ventricle, subsequently restoring the coaptation surface and preserving the cusp tissue (the idea is called „respect rather than resect“). Chordal replacement may be applied also in case of shortened native chord, that is resected and replaced by an arteficial one (of adequate length). To the advanced techniques belong: chordal transposition from one leaflet to another, papillary muscle repair, shaving of anular calcifications, augmentation of retracted leaflet by autologous pericardium, leaflet plications etc.
In cusp prolapse it is possible to operate on subvalvular apparatus, as well. Chordal shortening, or replacement by artificial goretex chords provide retraction of the prolapsed segments into the ventricle, subsequently restoring the coaptation surface and preserving the cusp tissue (the idea is called „respect rather than resect“). Chordal replacement may be applied also in case of shortened native chord, that is resected and replaced by an arteficial one (of adequate length). To the advanced techniques belong: chordal transposition from one leaflet to another, papillary muscle repair, shaving of anular calcifications, augmentation of retracted leaflet by autologous pericardium, leaflet plications etc.
A controversial technique with promising results, easy to perform, is Alfieri plasty (i.e. Edge-to-Edge repair, or „Double Mitral Orifice“). The principle is to suture prolapsed leaflets together in the middle part what leads to formation of two smaller orifices. It brings some risk of mitral valve stenosis in case of insufficient areas of the orifices, but on the other hand, it eliminates the risk of SAM.
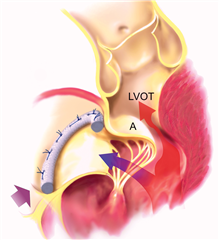 SAM (Systolic Anterior Motion) stands for anterior motion of the anterior leaflet of the mitral valve in systole. The leaflet is pulled by the blood flow into the left ventricular outflow tract what leads to its obstruction. At the same time the mitral valve regurgitation arises. The reason for this is a disproportion between excess leaflet material and smaller left ventricle, outflow tract and mitral anulus. Treatment for this condition is the volumotherapy and restriction of inotropes. As far as this is not effective, an immediate surgical correction is required.
SAM (Systolic Anterior Motion) stands for anterior motion of the anterior leaflet of the mitral valve in systole. The leaflet is pulled by the blood flow into the left ventricular outflow tract what leads to its obstruction. At the same time the mitral valve regurgitation arises. The reason for this is a disproportion between excess leaflet material and smaller left ventricle, outflow tract and mitral anulus. Treatment for this condition is the volumotherapy and restriction of inotropes. As far as this is not effective, an immediate surgical correction is required.
An essential part of valve-sparing procedures is echocardiographic control of the procedure outcome, still in the operating room, after restoring the heart activity. When the outcome of plasty is unsatisfactory, it is possible to stop the heart once again and attempt to improve it. Otherwise the valve can be replaced. Peroperative transoesophageal echocardiography can reliably detect possible procedural complications, as SAM, aortic valve impairment, damage to circumflex artery, drop in ejection fraction of the left ventricle.
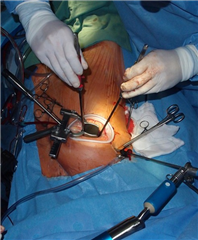 Nowadays the procedures of atrioventricular valves can be carried out through minimally invasive approach of right anterolateral thoracotomy, as well. This approach, apart from aesthetic benefit, provides some advantages for the patient especially in the short-term postoperatively - shorter artificial ventilation time, intensive care unit stay, faster rehabilitation and shorter hospital stay. Therefore, it should be preferred in suitable patients. The further step of miniinvasivity in mitral valve surgery is endoscopic valve plasty or robotically assisted plasty.
Nowadays the procedures of atrioventricular valves can be carried out through minimally invasive approach of right anterolateral thoracotomy, as well. This approach, apart from aesthetic benefit, provides some advantages for the patient especially in the short-term postoperatively - shorter artificial ventilation time, intensive care unit stay, faster rehabilitation and shorter hospital stay. Therefore, it should be preferred in suitable patients. The further step of miniinvasivity in mitral valve surgery is endoscopic valve plasty or robotically assisted plasty.
 In the context of minimizing the invasivity an interventional treatment of mitral valve regurgitation can be offered to high risk or inoperable patients. Via a catheter it is possible to perform a suture of mitral valve leaflets according to the principle of Alfieri plasty. The technology is called MitraClip, it is reserved for patients, who are not able to withstand the surgical operative burden and have suitable mitral valve anatomy. Another minimally invasive options include transapical implantation of artificial chords under ECHO control, transcatheter mitral valve anuloplasty or transcatheter implantation of the mitral valve. The latter is an analogue of TAVI, nevertheless, the outcomes are less satisfactory due to complexity of mitral valve apparatus.
In the context of minimizing the invasivity an interventional treatment of mitral valve regurgitation can be offered to high risk or inoperable patients. Via a catheter it is possible to perform a suture of mitral valve leaflets according to the principle of Alfieri plasty. The technology is called MitraClip, it is reserved for patients, who are not able to withstand the surgical operative burden and have suitable mitral valve anatomy. Another minimally invasive options include transapical implantation of artificial chords under ECHO control, transcatheter mitral valve anuloplasty or transcatheter implantation of the mitral valve. The latter is an analogue of TAVI, nevertheless, the outcomes are less satisfactory due to complexity of mitral valve apparatus.
Mitral valve replacement is the modality of choice in extensive valve disease where valve-sparing procedure is not possible. It is indicated in papillary muscle rupture after myocardial infarction and in severe destruction of the valve by infective endocarditis. Mechanical and biological prostheses (after 70 years of age) come into consideration.
RESULTS
Mitral valve replacement is connected with early mortality of 5-9%. Long-term survival is similar in patients with mechanical and biological prosthesis, it is around 50-60% in 10 years postoperatively. Patients with mechanical prosthesis are at risk of bleeding and thrombembolic complications in the long run. Patients with bioprosthesis are affected by a structural deterioration of the biological valve (around 10% in 15 years in population over 70 years of age).
In contrast, mitral valve plasty has excellent short- and long-term outcomes. Short-term mortality is currently <1% and 10-year survival is 70-85%. The patient is free from the risks of prosthetic material. The risk of re-do surgery (because of plasty failure) is comparable or lower as the risk of re-do in mechanical prosthesis. Long-term benefit strongly depends on the character and etiology of the regurgitation.
Tricuspid Valve Disease - Etiology, Pathogenesis, Diagnosis
Tricuspid valve stenosis is a rare disease. It may be caused by rheumatic process, carcinoid or cardiac tumors. By restriction of transtricuspid blood flow the blood stagnates and the pressure in the right atrium rises. The signs of right heart failure are present - systemic venous congestion (increased filling of the jugular veins, pleural effusion, hepatic enlargement, ascites and peripheral edema), low cardiac output (weakness, fatigue). In auscultation we find a mesodiastolic murmur above the bottom part of the sternum, opening snap may be present.
Tricuspid valve regurgitation is mostly of secondary etiology in pulmonary hypertension. A result of pressure- or volume-overload of the right ventricle is its hypertrophy and dilation, including the dilation of tricuspid valve anulus. The primary reason for this is mostly left-heart valve disease, pulmonary stenosis or regurgitation, congenital anomalies with left-to-right shunting, chronic obstructive pulmonary disease or pulmonary thrombembolism. A myocardial infarction or other right ventricular myocardial damage can lead to secondary tricuspid valve regurgitation, too.
Primary tricuspid regurgitation is less common and includes the following: myxomatous degeneration, rheumatic disease, infective endocarditis, trauma, carcinoid, congenital defects (Ebstein anomaly, various forms of atrioventricular septal defect), iatrogenic lesions.
Tricuspid valve regurgitation leads to volume-overload of the right atrium and right ventricle with subsequent right heart failure and corresponding symptoms. Atrial fibrillation often occurs. In the late stage are the patients cachectic, icteric, with cyanosis. Characteristic finding is pansystolic murmur above the bottom part of the sternum.
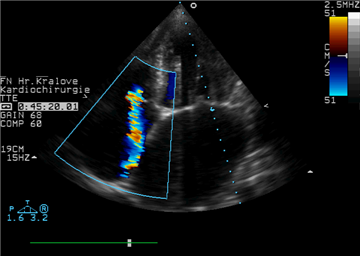 The preferred diagnostic modality is echocardiography. It serves to assess the presence, severity, etiology and mechanism of the valve disease. Tricuspid valve stenosis is considered severe with transtricuspid diastolic gradient >5 mmHg and valve orifice area <1 cm2. Severe tricuspid valve regurgitation is manifested as leaflet coaptation defect with eventual prolapse, severe regurgitation jet on Doppler imaging, systolic blood flow reversal in hepatic veins, vena contracta >7 mm, regurgitation orifice area >40 mm2, regurgitation volume >45 ml.
The preferred diagnostic modality is echocardiography. It serves to assess the presence, severity, etiology and mechanism of the valve disease. Tricuspid valve stenosis is considered severe with transtricuspid diastolic gradient >5 mmHg and valve orifice area <1 cm2. Severe tricuspid valve regurgitation is manifested as leaflet coaptation defect with eventual prolapse, severe regurgitation jet on Doppler imaging, systolic blood flow reversal in hepatic veins, vena contracta >7 mm, regurgitation orifice area >40 mm2, regurgitation volume >45 ml.
To assess the function of the right ventricle is the echocardiography only partially suitable. There are some ECHO-methods to quantify its function (e.g. TAPSE - tricuspid anular peak systolic excursion). Nevertheless, the best tool for this is currently the magnetic resonance imaging.
Tricuspid Valve Disease - Surgery
INDICATIONS
A standalone tricuspid valve surgery is nowadays relatively rare, it represents 5-10% of all tricuspid valve procedures. It is justified in patients with symptomatic right heart failure, in patients with progressive dilation of right heart chambers and impairment of right ventricular contractility. A special indication is an uncontrolled sepsis or heart failure in infective endocarditis of the tricuspid valve.
In the vast majority of cases is the tricuspid valve surgery concomitant to other cardiac procedures (mostly the valves of the left heart). Tricuspid valve surgery is always to proceed in severe tricuspid valve regurgitation or stenosis. Moderate tricuspid valve regurgitation in indicated for surgery in case of anular dilation or right heart failure. It has been proved that concomitant tricuspid valve repair does not increase the surgical risk and it offers a long-term benefit for the patient (lower mortality, morbidity, lower risk of valve disease recurrence, lower risk of re-do surgery).
PROCEDURES IN TRICUSPID VALVE STENOSIS
In suitable anatomy of rheumatic tricuspid valve disease is the valve-sparing procedure justified - incision of commissural adhesions. In specific cases it is possible to avoid surgery and perform the balloon plasty only. In extensive valve disease the valve replacement is indicated. Similarly as in mitral valve, there is an effort to preserve the septal and posterior leaflet. The valve of choice is usually the bioprosthesis, because in the low-pressure system is the degeneration significantly slower and there is no need for anticoagulation treatment. Moreover, it is possible to pass pacemaker electrodes through it.
PROCEDURES IN TRICUSPID VALVE REGURGITATION
The preferred method is valve-sparing tricuspid valve surgery - a plasty. Most popular is the implantation of anuloplastic ring that stabilizes the anulus and reduces its size to a desired value. It has usually a shape of letter „C“ - in the gap part is the atrioventricular bundle (of His) that could get injured by pressure / stitches. An alternative to the ring anuloplasty is a suture anuloplasty sec De Vega which shows slightly worse long-term outcomes. The most simple option is the bicuspidalization of the tricuspid valve sec Kay-Reed. It consist in oversewing the posterior leaflet with a pledgeted suture - the leaflet is practically disabled and anulus gets smaller. The „triple orifice“ technique (a modification of Alfieri plasty for tricuspid valve) was described too - the leaflets are sutured together in the middle part and a shape of an imaginary shamrock is formed. The valve replacement comes into consideration in extensive valve disease (e.g. in infective endocarditis).
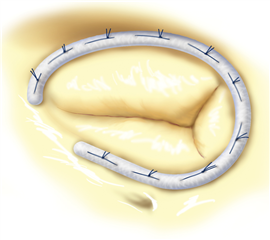
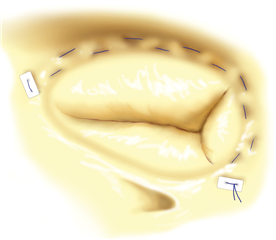
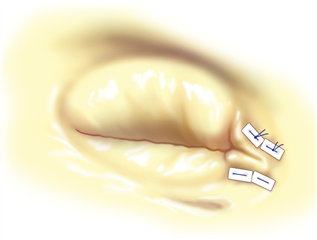
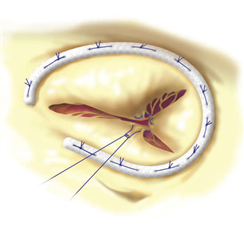
In specific cases a plain tricuspid valve excision is possible. The blood flow to the ventricle is not interrupted but massive regurgitation remains. This technique may be functional only without pre-existing pulmonary hypertension and with intensive volumotherapy in the postoperative period to preserve high right atrial pressure. This procedure can be considered only in intravenous drug abusers with infective endocarditis of tricuspid valve where there is a risk of re-infection in postoperative period. Alternatively a homograft could be implanted - the material is less prone to infection but the implantation procedure is technically very demanding.
Resources:
- Dominik J. Kardiochirurgie. Praha: Grada Publishing, 1998. ISBN: 80-7169-669-2
- Cohn LH, Adam DH. Cardiac Surgery in the Adult, Fifth Edition. New York: McGraw Hill Education, 2016. ISBN 978-0-07184487-1.
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm Ch, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2018; 38: 2739-2791. doi:10.1093/eurheartj/ehx391
- Dominik J, Žáček P. Chirurgie srdečních chlopní (... nejen pro kardiochirurgy). Praha: Grada Publishing, a.s., 2008. ISBN: 978-80-247-6386-6.
- Češka R a kol. Interna. Praha: Triton, 2010. ISBN: 978-80-7387-423-0.
- Schäfers HJ. Current Treatment of Mitral Rregurgitation. Bremen: UNI-MED-Verlag, 2010. ISBN: 978-3-8374-1211-6.
- Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim S, Mishell JM et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. New England Journal of Medicine 2018; 379: 2307-2318. doi: 10.1056/NEJMoa1806640.
